UK scientists are to carry out studies on a new “breakthrough” blood test which could be used to detect certain types of brain cancer.
Described as a “liquid biopsy”, the test, believed to be a world-first, could reduce the need for invasive and risky surgery currently needed to diagnose some brain tumours.
The tests could also lead to an earlier diagnosis, which in turn could speed up treatment and potentially increase survival rates for patients with one of the deadliest forms of brain cancer, experts say.
Patients with inoperable brain tumours could particularly benefit – because an early diagnosis would allow them to start treatments such as chemotherapy or radiotherapy as soon as possible.
Scientists in the UK have already studied the tests and are now hoping to conduct larger trials.
If successful, the “inexpensive” and “patient-friendly” test could be rolled out to health services within two years, experts say.
What is the test?
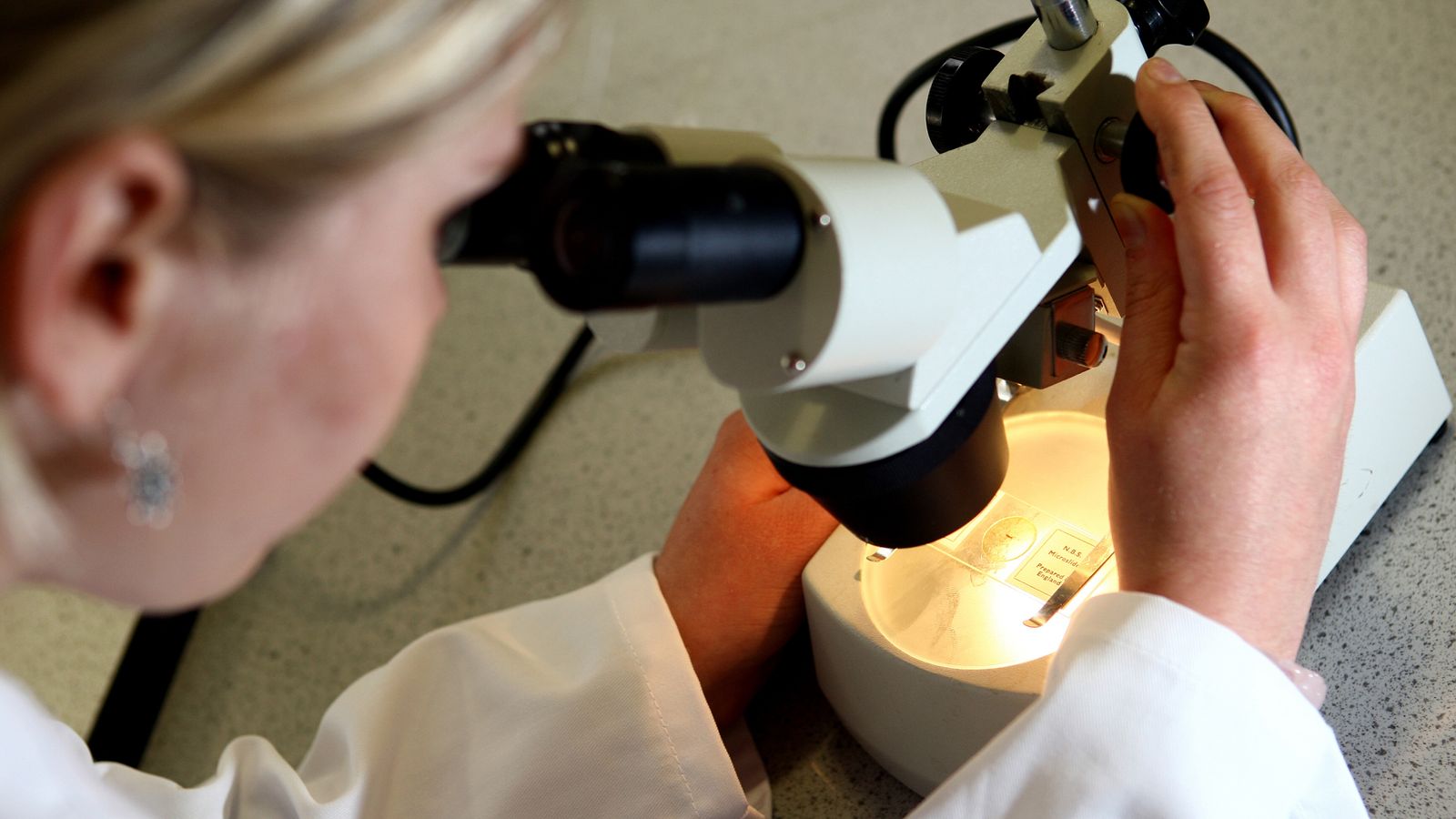
The TriNetra-Glio blood test, developed by Datar Cancer Genetics, works by isolating cells that have broken free from the tumour and into a person’s blood.
The isolated cells are then stained and can be identified under a microscope.
The test has been studied by researchers at the Brain Tumour Research Centre, which is run by Imperial College London and Imperial College Healthcare NHS Trust.
They have performed the first studies to assess whether the test can accurately diagnose glial tumours – which develop in the brain and can grow to press on the brain or spinal cord tissue.
These include glioblastoma (GBM) – the most commonly diagnosed type of high-grade brain tumour in adults – astrocytomas, and oligodendrogliomas.
They found the test had “high analytical sensitivity, specificity and precision”, according to a study published in the International Journal of Cancer.
Scientists now hope to conduct larger studies in the UK to validate the results.
‘A real breakthrough’
Dr Nelofer Syed, who leads the Brain Tumour Research Centre of Excellence at Imperial, said: “A non-invasive, inexpensive method for the early detection of brain tumours is critical for improvements in patient care.
“There is still some way to go, but this solution could help people where a brain biopsy or surgical resection of the tumour is not possible due to the location of the tumour or other constraints.
“Through this technology, a diagnosis of inaccessible tumours can become possible through a risk-free and patient-friendly blood test.
“We believe this would be a world first as there are currently no non-invasive or non-radiological tests for these types of tumours.”
Kevin O’Neill, consultant neurosurgeon at Imperial College Healthcare and honorary clinical senior lecturer at Imperial College London, who leads the Brain Tumour Research Centre of Excellence alongside Dr Syed, said: “This test is not just an indicator of disease, it is a truly diagnostic liquid biopsy.
“It’s a real breakthrough for treatment of brain cancers that rarely spread around the body.
“This could help speed up diagnosis, enabling surgeons to apply tailored treatments based on that biopsy to increase patients’ chances of survival.”
‘We have to find a cure’
Brain Tumour Research said that the findings are “significant” as less than 1% of patients with GBM live for more than 10 years and, for many, the prognosis is as little as 12 months.
Be the first to get Breaking News
Install the Sky News app for free
Read more from Sky News:
Shoplifting offences rise to highest level in 20 years
Chief vet urges XL bully owners to register dogs ahead of ban
Dan Knowles, chief executive of the charity, said: “This ground-breaking research could lead to earlier diagnosis and improved outcomes for brain tumour patients.
“Brain tumours kill more people in the UK under the age of 40 than any other cancer and we have to find a cure for this devastating disease.
“It is scandalous to think that there have been no improvements to treatment options for this type of tumour in two decades and the standard of care for GBM patients – surgical resection followed by radiotherapy and chemotherapy – remains unchanged.”